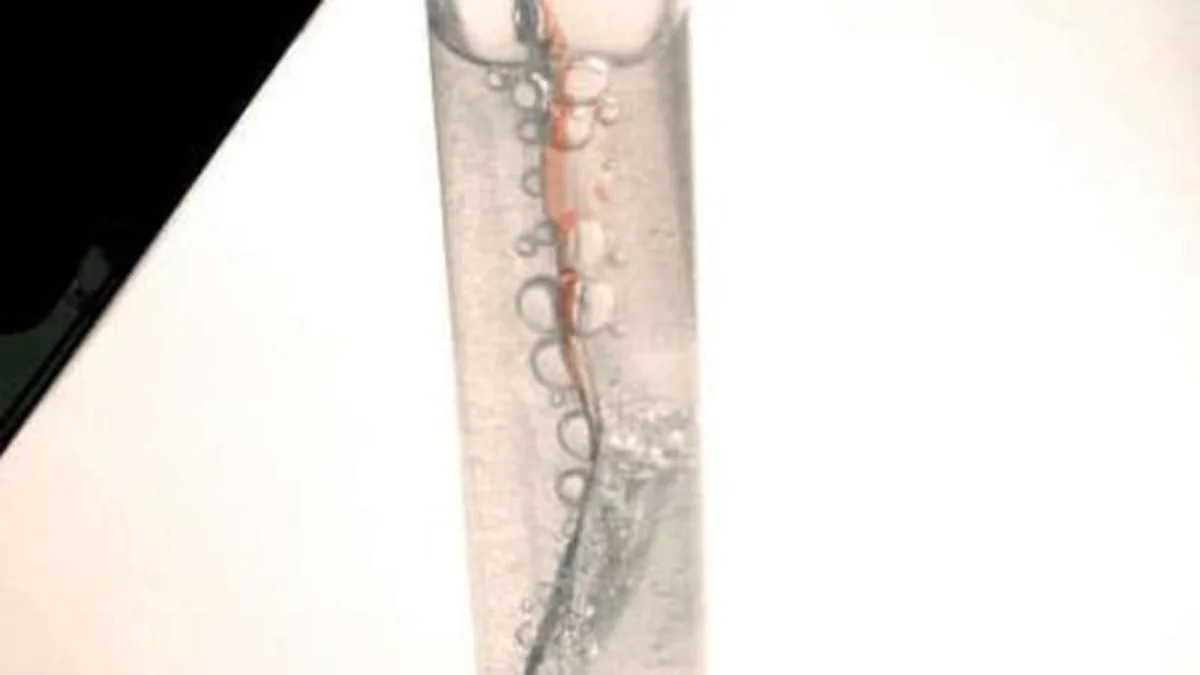
Do you remember when we told you about the scientists from Purdue who were working on a technique to produce hydrogen on-demand from aluminum using gallium and water? It seems that there are some enterprising individuals who were intrigued by the work of Dr. Woodall's experiments. Take a look at this Instructable, which teaches you how to capture some hydrogen using a similar technique, this time using strips of aluminum from soda cans covered with a "liquid metal" which are then immersed in water. The reaction that takes place releases hydrogen at a rapid rate, according to the article. We're not sure that this process is cost effective or practical for large-scale capture of hydrogen, but we do think that the technique is interesting enough to share.
Note that the comments for the article indicate that the gallium is recoverable. Any aluminum used will be lost, as it is the reaction of the aluminum and water which releases the hydrogen.
[Source: Instructable]

Sign in to post
Please sign in to leave a comment.
Continue