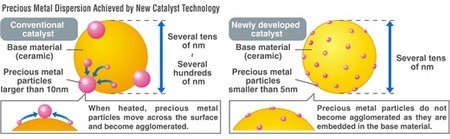
One of the keys to cutting vehicle emissions over the past 40 years, aside from electronic engine management systems, has been the catalytic converter. Unfortunately, catalytic converters are expensive due to the fact that precious metals such as platinum and rhodium have to be used to trigger the reactions that transform the noxious emissions from the engine into something less harmful. Traditional converter manufacturing methods use base material coated with the catalyst. Unfortunately, the catalyst tends to clump up, reducing the effective surface area that makes contact with the exhaust.
Mazda has developed a new manufacturing process with a base structure that holds the precious metal catalyst material in place. When combined with a method of producing nano-sized catalyst particles less than 5nm in diameter, the effective area that is exposed is dramatically increased. Mazda has managed to reduce the amount of precious metals in the converter for the new 2010 Mazda3 by 70 percent to just 0.15g/L of capacity. Given the spike in precious metal prices in the past year, this could be a huge benefit to Mazda and whoever they share the technology with. If the same technology can be applied to other types of catalytic converters like lean NOx traps, it could also reduce the cost premium of diesel engines.
[Source: Mazda]
PRESS RELEASE:
Mazda Cuts Precious Metal Usage 70 Percent in New Single-nanocatalyst
HIROSHIMA, Japan-Mazda Motor Corporation has announced the world's first market application of single-nanocatalyst* technology in automobile catalytic converters. This highly durable new catalyst significantly reduces the amount of precious metals used and effectively purifies vehicle exhaust gases. It will first be introduced in the all-new Mazda3 (known as the Mazda Axela in Japan) which will commence global sales this year.
With the single-nanocatalyst, the underfloor catalytic converter in the all-new Mazda3 requires only 0.15g/L of precious metals, approximately 70 percent less than the 0.55g/L required in the previous model. Along with the substantial reduction in precious metal usage, the Mazda3 continues to qualify as a Super Ultra-Low Emissions Vehicle (SU-LEV) in Japan by achieving exhaust emissions that are at least 75 percent cleaner than the government's 2005 regulations.
Automotive catalysts consist of a base material coated with precious metal particles. These metals promote chemical reactions that purify exhaust gases. In conventional catalysts, exposure to hot exhaust gases causes the precious metal particles to agglomerate into larger clumps, which reduces their effective surface area and catalytic activity. To counteract this, an increased amount of the precious metals is required to maintain an efficient purification performance.
Mazda developed the single-nanocatalyst to increase the effective surface area of the precious metals used. By developing a method of controlling precious metal particles that are less than five nanometers (nm) in diameter as well as a proprietary catalyst material structure, Mazda created the world's first catalyst that features single-nanosized precious metal particles embedded in fixed positions. As a result, there is no agglomeration of the particles and the amount of rare metals used can be significantly reduced. Moreover, the new catalyst material will enable very efficient purification with minimal deterioration over time even under the harshest operating conditions.
Going forward, Mazda will progressively introduce the single-nanocatalyst to all its global markets, which will contribute to a reduction in the consumption of rare metals and cleaner vehicle exhaust emissions.

Sign in to post
Please sign in to leave a comment.
Continue