Over the past decade, most of the world's major automakers have expended a lot research dollars and engineering resources on developing vehicles that burn hydrogen. While advocates like the idea of using hydrogen as an energy carrier because it's the most abundant element in the known universe and it can be used without emitting toxic or greenhouse gas emissions (disregarding – for the moment – any emissions from producing the hydrogen), not everyone agrees how to use it. There are two basic approaches to using hydrogen in vehicles: the proton exchange membrane (PEM, also called polymer electrolyte membrane) fuel cell and the classic internal combustion engine (ICE).
While some automakers, notably Ford, have experimented with both approaches, most OEMs have chosen one direction or the other. Aside from Ford, the only other automakers making any significant effort with hydrogen ICEs are BMW and Mazda. Virtually everyone else working with hydrogen has gone fuel cell. Why go one direction or the other? Read on after the jump to find out.
Hydrogen ICEs
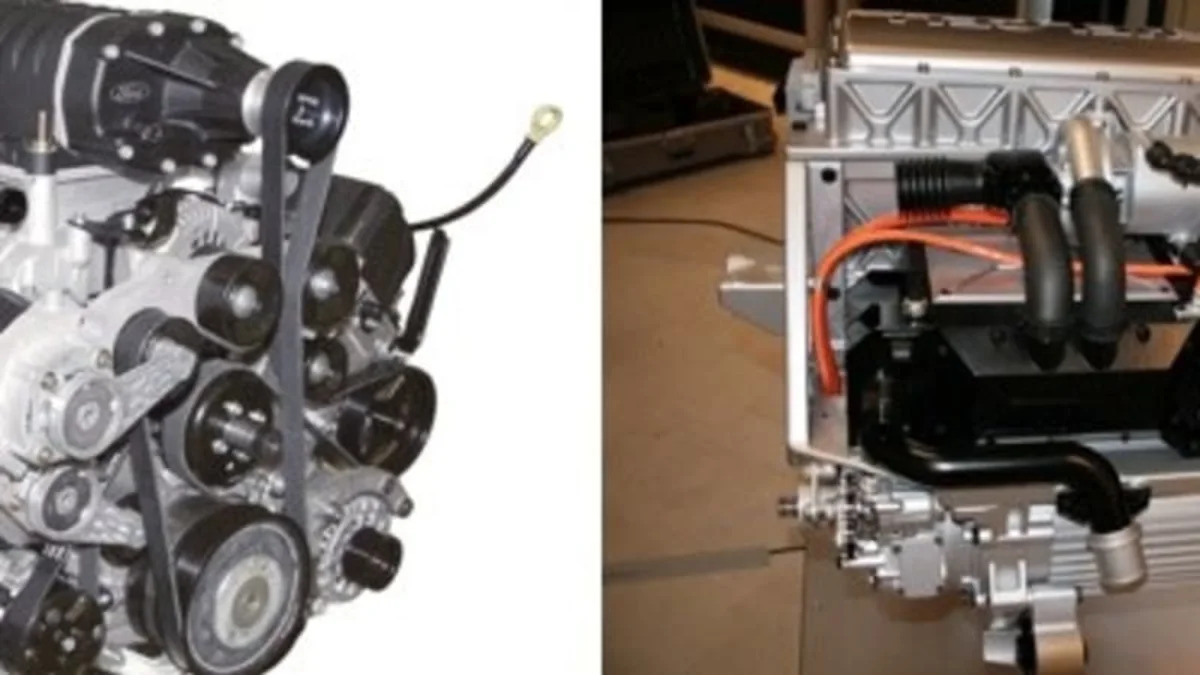
The primary reason for using hydrogen in internal combustion engines is that they already exist and are comparatively inexpensive. Since hydrogen combusts fairly readily, it doesn't take much in the way of modifications - mainly new fuel injectors and a storage system - to make hydrogen work in an ICE. This is, of course, a bit of an oversimplification. While the basics are the same, the combustion properties of hydrogen are very different from gasoline or diesel. It burns much faster than those fuels, so getting the most out of hydrogen in an ICE requires optimizing the shape of the combustion chamber and calibrating the timing of the spark in order to avoid damaging knock.
Ford and BMW have both pursued hydrogen ICEs using traditional piston engines. BMW has gone further and actually uses liquid hydrogen as a fuel while virtually every other automaker has focused on compressed gaseous hydrogen. BMW has also built a run of 100 7-series sedans (above) that are actually dual fueled with the ability to run on either hydrogen or gasoline. Mazda, as it so often does, has followed a different path, choosing to use it's Wankel rotary engines as the basis for its hydrogen ICE work. The Wankel is better suited to running on hydrogen for a number of reasons having to do with the lack of valves that can create hot-spots for spontaneous combustion and the ability to use ceramic seals to avoid lubrication issues. We'll tackle those issues in another article.
There are some downsides to using hydrogen in ICEs. While hydrogen burning in oxygen yields nothing but water, air is only 20 percent oxygen. Most of the rest is nitrogen. Burning hydrogen in air produces trace amounts of nitrogen oxides (a tiny fraction of what is produced when burning gasoline or diesel). The other problem is power output. While hydrogen has higher mass energy density than gasoline (143MJ/kg vs. 46.4) its volumetric density tends to be very low. As a result, while gasoline has an energy density of 34.2 MJ/L, liquid hydrogen is only 10.1 MJ/L and compressed gaseous hydrogen (700 BAR) is only 5.6 MJ/L. That means ICEs tend to produce a lot less power on hydrogen than they do on gas. Ford combated this on its hydrogen V10 engine by supercharging it, making up some of the deficit.
On to the H2 fuel cell
The preferred approach to using hydrogen long term is the fuel cell. Fuel cells use a process that is essentially the reverse of electrolysis to combine hydrogen and oxygen in the presence of a catalyst to generate electricity. The only by-product of the process is water. Fuel cells are much more efficient than ICEs, often topping 70 percent. The main problem with fuel cells is the cost. Until now, fuel cells have been largely hand made one at a time which greatly increases the cost of manufacturing. Now that the basic technology premise of fuel cells has been demonstrated, automakers are working to design fuel cell stacks that integrate ancillary systems such as cooling, water drainage and fuel delivery. These units, such as GM's 5th generation stack, are designed to be mass produced on automated equipment at much lower cost.
The other cost factor for fuel cells is the catalyst. The plates that make up the working part of a fuel cell stack are coated with platinum, which is of course very expensive. Most automakers are reluctant to give details of the internals of their stacks but GM recently revealed that its fourth generation fuel cell stack used in the Chevy Equinox for Project Driveway contained 80 grams of platinum. Its next-generation stack contains only 30 grams and the upcoming iteration is expected to need less than 10 grams, putting it on a par with catalytic converters.
Besides cost, the other primary issue with fuel cells is durability. Early stack designs had short lifespans caused in part by "poisoning" of the catalyst from impurities in the hydrogen and air supplies. Newer designs have overcome some of this and made the plates more resistant to reacting with those impurities. Because water is the by-product of the reaction, drainage is critical, and early stack designs had issues with cold weather operation. Newer designs have addressed many of these problems and the latest stacks can now start and operate at temperatures down to -30 F. GM's Project Driveway fuel cells are now approaching 80,000 miles of durability and could go much higher before the program ends.
Hydrogen continues to hold promise as a future transportation fuel, especially if it can be produced from renewable sources. However, before either hydrogen ICEs or H2 fuel cells can make any significant in-roads, a publicly-accessible distribution network needs to be in place – and that is happening at an exceedingly slow pace.
Car Buying Resources
While some automakers, notably Ford, have experimented with both approaches, most OEMs have chosen one direction or the other. Aside from Ford, the only other automakers making any significant effort with hydrogen ICEs are BMW and Mazda. Virtually everyone else working with hydrogen has gone fuel cell. Why go one direction or the other? Read on after the jump to find out.
Hydrogen ICEs
The primary reason for using hydrogen in internal combustion engines is that they already exist and are comparatively inexpensive. Since hydrogen combusts fairly readily, it doesn't take much in the way of modifications - mainly new fuel injectors and a storage system - to make hydrogen work in an ICE. This is, of course, a bit of an oversimplification. While the basics are the same, the combustion properties of hydrogen are very different from gasoline or diesel. It burns much faster than those fuels, so getting the most out of hydrogen in an ICE requires optimizing the shape of the combustion chamber and calibrating the timing of the spark in order to avoid damaging knock.
Ford and BMW have both pursued hydrogen ICEs using traditional piston engines. BMW has gone further and actually uses liquid hydrogen as a fuel while virtually every other automaker has focused on compressed gaseous hydrogen. BMW has also built a run of 100 7-series sedans (above) that are actually dual fueled with the ability to run on either hydrogen or gasoline. Mazda, as it so often does, has followed a different path, choosing to use it's Wankel rotary engines as the basis for its hydrogen ICE work. The Wankel is better suited to running on hydrogen for a number of reasons having to do with the lack of valves that can create hot-spots for spontaneous combustion and the ability to use ceramic seals to avoid lubrication issues. We'll tackle those issues in another article.
There are some downsides to using hydrogen in ICEs. While hydrogen burning in oxygen yields nothing but water, air is only 20 percent oxygen. Most of the rest is nitrogen. Burning hydrogen in air produces trace amounts of nitrogen oxides (a tiny fraction of what is produced when burning gasoline or diesel). The other problem is power output. While hydrogen has higher mass energy density than gasoline (143MJ/kg vs. 46.4) its volumetric density tends to be very low. As a result, while gasoline has an energy density of 34.2 MJ/L, liquid hydrogen is only 10.1 MJ/L and compressed gaseous hydrogen (700 BAR) is only 5.6 MJ/L. That means ICEs tend to produce a lot less power on hydrogen than they do on gas. Ford combated this on its hydrogen V10 engine by supercharging it, making up some of the deficit.
On to the H2 fuel cell
The preferred approach to using hydrogen long term is the fuel cell. Fuel cells use a process that is essentially the reverse of electrolysis to combine hydrogen and oxygen in the presence of a catalyst to generate electricity. The only by-product of the process is water. Fuel cells are much more efficient than ICEs, often topping 70 percent. The main problem with fuel cells is the cost. Until now, fuel cells have been largely hand made one at a time which greatly increases the cost of manufacturing. Now that the basic technology premise of fuel cells has been demonstrated, automakers are working to design fuel cell stacks that integrate ancillary systems such as cooling, water drainage and fuel delivery. These units, such as GM's 5th generation stack, are designed to be mass produced on automated equipment at much lower cost.
The other cost factor for fuel cells is the catalyst. The plates that make up the working part of a fuel cell stack are coated with platinum, which is of course very expensive. Most automakers are reluctant to give details of the internals of their stacks but GM recently revealed that its fourth generation fuel cell stack used in the Chevy Equinox for Project Driveway contained 80 grams of platinum. Its next-generation stack contains only 30 grams and the upcoming iteration is expected to need less than 10 grams, putting it on a par with catalytic converters.
Besides cost, the other primary issue with fuel cells is durability. Early stack designs had short lifespans caused in part by "poisoning" of the catalyst from impurities in the hydrogen and air supplies. Newer designs have overcome some of this and made the plates more resistant to reacting with those impurities. Because water is the by-product of the reaction, drainage is critical, and early stack designs had issues with cold weather operation. Newer designs have addressed many of these problems and the latest stacks can now start and operate at temperatures down to -30 F. GM's Project Driveway fuel cells are now approaching 80,000 miles of durability and could go much higher before the program ends.
Hydrogen continues to hold promise as a future transportation fuel, especially if it can be produced from renewable sources. However, before either hydrogen ICEs or H2 fuel cells can make any significant in-roads, a publicly-accessible distribution network needs to be in place – and that is happening at an exceedingly slow pace.
Car Buying Resources

Sign in to post
Please sign in to leave a comment.
Continue